Recombinant FGF-2 TOP® (Thermostability Optimized)
Animal component-free, Endotoxin-free, Biorisk-free / Next-generation Growth Factors for Stem Cells and Cell-based applications
| FGF-2-TOP® (Thermostability Optimized) -formerly STAB- is a stabilized growth factor that offers a novel way to grow FGF-2-dependent cell cultures more efficiently, with fewer media changes. FGF2-TOP® retains full biological activity even after seven days at 37°C. The stable levels of FGF-2 in culture allows for a more homogenous, undifferentiated stem cell culture, while saving researchers valuable time and money because repeated supplementation with FGF-2 and a daily medium change is not required. FGF-2 TOP® is the 155 aa mature domain of FGF-2 with nine amino acid substitutions to enhance stability without impacting bioactivity developed by Dvorak et al. 2018. This increases the functional half-life of the protein from <10 h (wild-type) to >7 days (FGF2-TOP®) in cell culture conditions at 37ºC. |  |
Product Overview
FGF-2 TOP® recombinant protein is derived from a proprietary, plant-based production system that ensures high purity and sustainability. Completely animal component-free, it is a safer and more ethically sourced choice for stem cell research and cell therapy applications
Uniquely Engineered for Stem Cell Production:
Through novel protein engineering, FGF-2 TOP® offers an increased half-life and therefore fewer feedings required compared to the wild-type growth factor.
It is significantly more tolerant of heat while maintaining full bioactivity, leading to improved homogeneity in your cell growth. Derived from our proprietary plant-based production system, FGF-2 TOP® is also fully animal component-free, endotoxin-free, and sustainably produced.

Product Information:
|
Alternative Names
|
FGF-2 G3, bFGF STAB, Thermostable FGF-2
|
|
Amino Acid Sequence
|
154 amino acids with 9 aa point mutations from the wild-type human FGF-2. Tag-free. Original reference sequence accession number: P09038 |
|
Molecular mass
|
Estimated 17.4 kDA |
|
Origin
|
Plant derived (Camelina Sativa)
|
|
Species
|
Engineered sequence
|
|
Similarity
|
Species neutral. Reactivity with Human, Bovine, Porcine and Mouse |
Specifications:
|
Purity
|
95% SDS page resolved under reduced (R) conditions. |
|
Bioactivity
|
EC50 ≤ 0.5 ng/ml |
|
Formulation
|
Lyophilized |
|
Endotoxin levels
|
Recombinant protein expressed in plant system, free of bacterial endotoxins. |
|
Animal / Human components
|
Free
|
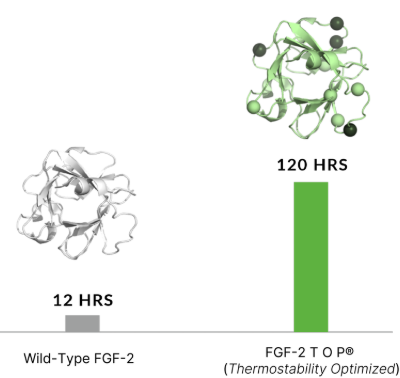
Increased Thermostability
In order to maintain stem cell homogeneity and avoid spontaneous differentiation, scientists traditionally had to maintain a strict daily feeding schedule due to the short half-life (approximately 9 hours at 37°C) and temperature sensitivity of wild-type FGF-2.
| Increased half-life: 10-fold improvements at 37°C | Supplement Less and Achieve More Consistency |
 |
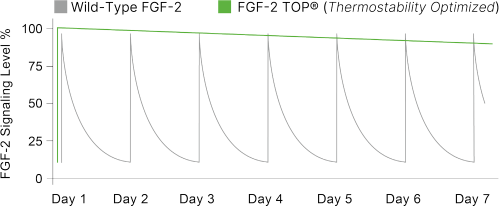 |
| To improve stability, a novel nine amino acid substitution of the wild-type FGF-2 was performed, improving the heat stability of FGF-2 TOP resulting in an increase in half-life under cell culture conditions to 37°C. | By nature of its increased half-life and stability, FGF-2 TOP presents a constant exposure of growth factor (green line) in contrast to the short half-life and signaling of the wild-type protein that must be replenished daily (grey line). Thus, feeding schedules are more streamlined and cell culture phenotype is more homogenous. |
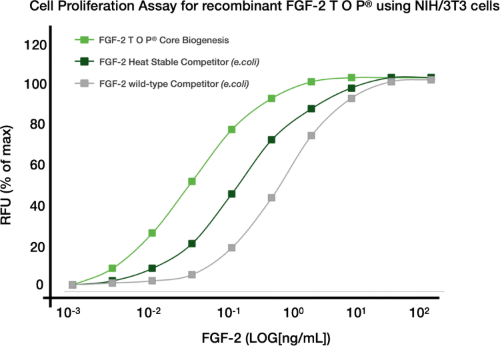
Superior Bioactivity
|
Retained Superior Bioactivity Comparison of FGF-2 TOP®with a FGF-2 heat stable competitor and human FGF-2 wild-type in a 3T3 cell proliferation assay using varying concentrations of each protein after a 48-hour incubation at 37°C. FGF-2 TOP demonstrates maintenance of full bioactivity and with a 5-fold lower EC50, FGF-2 TOP® demonstrates a greater capacity to promote 3T3 cell proliferation and at lower concentrations than competing heat stable alternatives and wild-type FGF-2 in this model. |
 |
|
Supporting Proliferation of Adherent Cell Culture In an internal MSC model analyzing confluence over 9 days, the addition of FGF-2 TOP® to an internal MSC formulation resulted in an equivalent cell confluence and expansion (as assessed through cell attachment) compared to the positive control containing 10% FBS. Similar results have been observed in both iPSC and adherent HEK-293 models (assessed but not shown). |
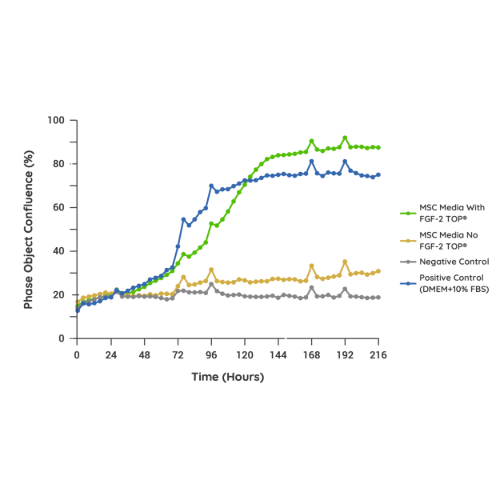 |
|
Maintains Pluripotency and Stemness FGF-2 TOP® maintains high-quality iPSC cultures under various concentrations and media feeding regimes as assessed by flow cytometry. Cells were treated with wild-type “human” FGF-2 or FGF-2 TOP® at 50 or 100 ng/mL either daily if not indicated or every other day (EOD). The negative control contained no FGF-2 supplementation. All tested conditions resulted in high expression of pluripotency markers. Most notably, EOD feeding with FGF-2 TOP® showed equivalent levels as well as provided further proof of bioactivity maintenance after stability changes. |
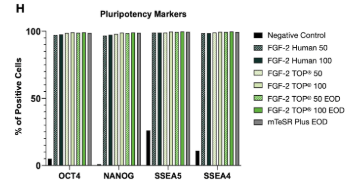 |
Weekend-Free Feeding
Realize tangible benefits of FGF-2 TOP® protein stability: significant reductions in the amount of media required to feed cells, as well as reductions in the number of feedings, saving labor costs and obviating the need for inconvenient weekend feedings. Refer to the example feeding schedule and cost comparison of wild-type versus TOP®:
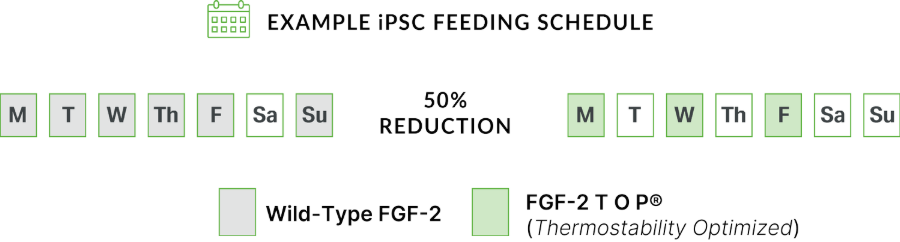
The Performance to Optimize Your PSC Cultures & Differentiation
|
Intensified iPSCs expansion FGF-2 TOP® provides superior performance for high-quality iPSC cultures. A) When iPSCs are cultured in adherent conditions (2D) and supplemented with 100ng/mL of FGF-2 TOP®, cells display typical colony-forming morphologies, preventing the appearance of differentiated cells. B) When iPSCs are expanded in suspension (3D) and supplemented with 100ng/mL of FGF-2 TOP®, cells form embryoid bodies (EBs) with consistent shape and size. |
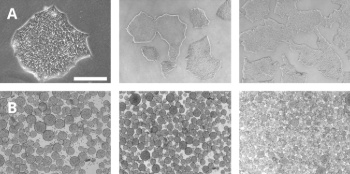 |
|
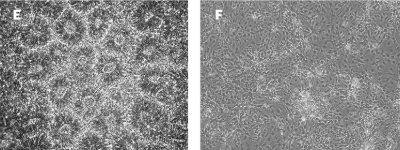
Robust differentiation into NSC phenotypes FGF-2 TOP® supports differentiation of iPSCs into Neural Stem Cells. E) Neural rosettes are formed from dissociated EBs when supplemented with 20ng/mL of FGF-2 TOP®. F) Cells transferred from the neural rosettes into pO/Lam-coated plates, start to differentiate into Neural Progenitor Cells (NPCs) reaching 80-90% of confluence after culture with 20ng/mL of FGF-2 TOP® |
|
The Consistency for Your Results while Working with MSCs Cultures
|
Increased cell numbers during MSC maintenance Exogenous supplementation FGF-2 TOP® promotes higher increase of cell proliferation rates than “Wild-Type” FGF-2 on hMSCs. After 3 days in culture, hMSCs cultured with 10ng/mL of the wild-type version of hrFGF-2 (reference standard) promoted a cell expansion from 2x10^5 (seeding density) to 9.81x10^5. When hMSCs were supplemented with 10ng/mL of FGF-2 TOP® (Core Bio) cells expanded from the same seeding density to 18.42x10^5. |
 |
Publications
• FGF2-G3 was developed by Dvorak et al., 2018. Biotechnology and Bioengineering
• B8 media protocol established by Kuo et al., 2020. Stem Cell Reports
• updated in Lyra-Leite et al., 2021. STAR Protocols
• Review of FGF 2 stabilization by Benington et al., 2020. Pharmaceutics
• Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat, Kaplan et al., 2021.
Application Note and Case Study
Application Note:
"Evaluating the use of Thermostable FGF-2 (FGF-2 TOP®) for optimized iPSC Culture Protocols": This study highlights how the consistent signaling allows to obtain higher quality pluripotent stem cell cultures, while contributing to process intensification with higher cell yields, allowing to reduce the media feeding schedules, and increasing the expression of pluripotency markers.
Case Study:
"FGF-2 TOP® (Thermostability Optimized) enhances cell proliferation of MSCs and NHDFs": The results show how stable FGF-2 outperforms the wild-type version of FGF-2 (with a competitor comparison as well) both in the cell culture expansion and marker expression- improving the cell phenotype profiles.