EZSPHERE™ for 3D cell culture with microwells
Cell Culture ware for Spheroid Formation
| Three dimensional (3D) cell culture systems have gained in popularity as invaluable tools in broad applications of cell biology. 3D multi-cellular cell aggregates (Spheroid) can be formed by using a low attachment culture surface. However, variability in forming spheroids has been a persistent problem. EZSPHERE™ is specifically designed to form a large number of uniformly sized Spheroids and Embryoid Bodies (EBs). | .jpg) |
Features
- Microwells, created by CO2 gas laser and coated with low adhesive reagents (MPC polymer), generate a large number of spheroids and EBs with uniform size.
- Application for EBs formation and expansion of iPSCs
- Applicable to both adherent and suspension cell culture
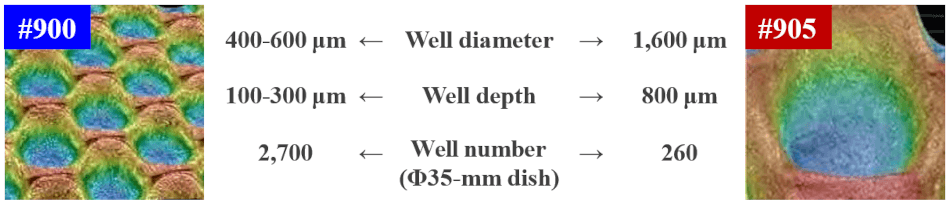
- Available in six micro-well sizes: EB size control with micro-well sizes or inoculating cell densities
| Micro well type |
Diameter |
Depth |
 |
|---|---|---|---|
| #901 | 250 | 100 | |
| #900 | 400~600 | 100~300 | |
| #902 | 450 | 200 | |
| #903 | 950 | 400 | |
| #904 | 1,000 | 350 | |
| #905 | 1,600 | 800 |
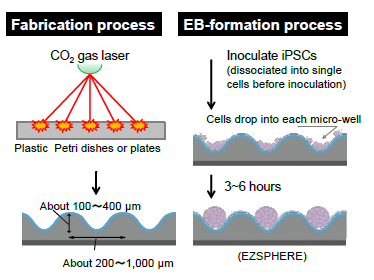
Unique micro-fabricated plastic vessel
EZSPHERE™ is specifically designed for creating a large number of spheroids and EBs with uniform size.
 |
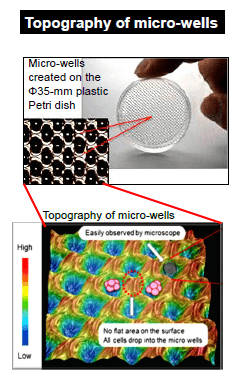 |
| Micro-wells of EZSPHERE™ are solely created by CO2 gas laser on the plastic dishes or plates, followed by coating with low adhesive reagents (MPC polymer). Because micro-wells are closely positioned each other in the plastic culture ware, inoculated cells equally drop into each well. |
EB formation, proliferation and differentiation protocols
Innovative culture mothod for iPSCs using the EZSPHERE™ : ZSPHERE™ can be used for dveloping large-scale and efficient iPSCs producing techniques.
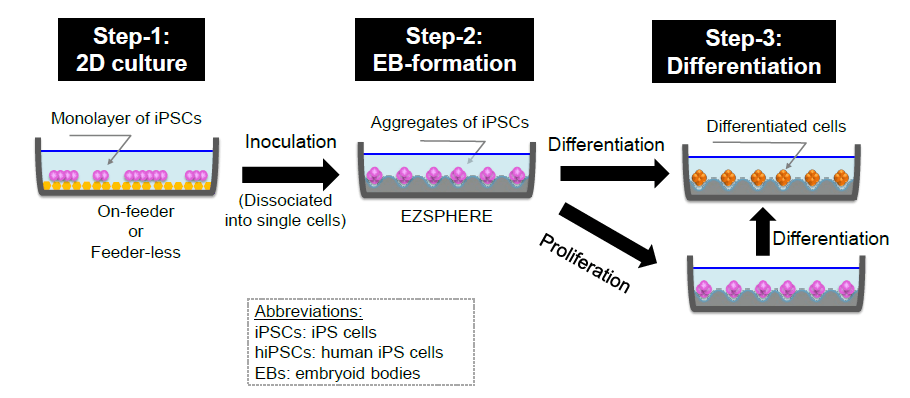
Step-1 : Proliferate hiPS cells with on-feeder or feeder-less condition.
Step-2 : Dissociate hiPS cells into single cells and inoculate them into EZSPHERE™, where inoculated cells equally drop into each micro-well and form aggregate “EBs” in 3 to 6 hours.
Step-3 : Proliferate hiPS cells in the EB form with un-differentiation medium. Differentiate the EBs with using differentiation medium.
Large amount of EBs with uniform size
High efficient generation of EBs with uniform size on the EZSPHERE™
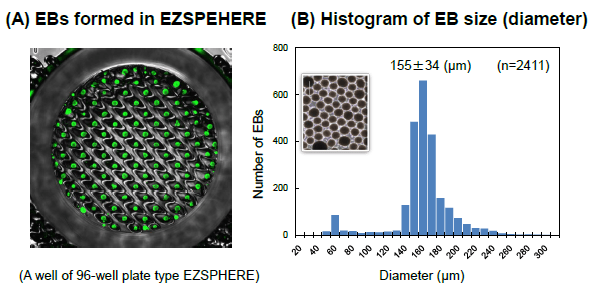
(A) Fluorescence microscopy image of EBs obtained ont he EZSPHERE™. 2D cultured iPScells were inoculated into the EZSPHERE™ after dissociating into single cells and stained with CalceinAM(greenforlivingcells ) next day.
(B) Histogram of EBsize (diameter) distribution. EBs created on the 35-mmΦDishtype EZSPHERE™ were imaged and analyzed with the digital image analyzing software “Image J” to determine size distribution. The Gaussian distribution of EB size indicated uniformly sized EBs in the EZSEHERE™.
EB-size control with micro-well sizes or inoculating cell densities
EB size is controlled by changing the inoculation cell number and/or micro-well size of EZSPHERE™
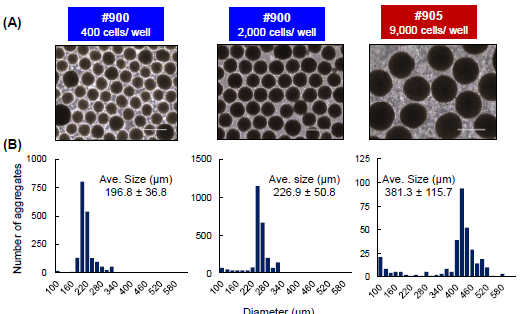
(A) Phase contrast image of iPSC aggregate generated from different number of iPSCs as 400 or 2,000 cells per micro-well in EZSPHERE™#900 or 9,000 cells per micro-well in Larger size of microwell EZSPHERE™#905 (diameter = 1,400 μm). Scale Bar = 400 μm
(B) The size of iPSC aggregate was uniform and could be controlled by inoculation number of iPSCs and/or micro-well size.
Expansion of hiPSC aggregates
iPSCs grown as EBs with feeder-free cell cultural medium on the EZSPHERE™ maintained their puripotency
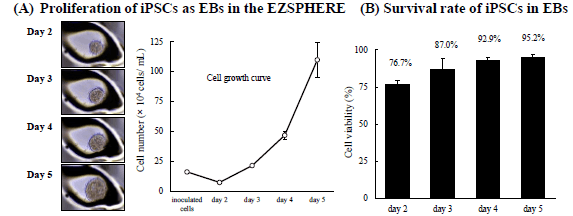
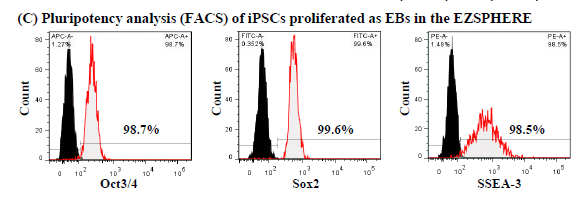
When iPSCs were cultured on EZSPHERE™ with feeder-free cell culture medium (mTeSR1), the formed EBs could proliferate at a good rate (A) with high viability (B). In the flow cytometry, these cells maintained high capacity of undifferentiated state (C).
Differentiation of hiPSC aggregates
1) Dopaminergic neuron differentiation in EZSPHERE™
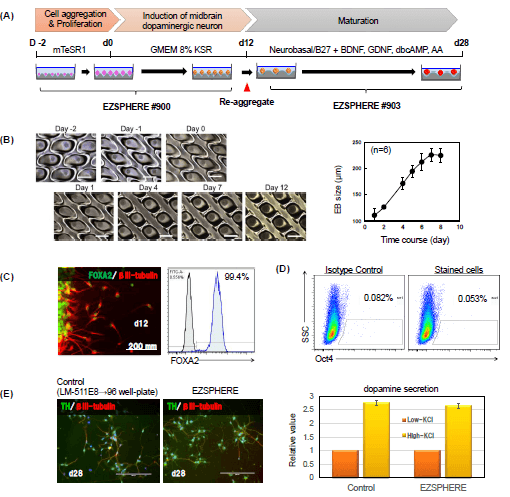
(A) Differentiation of hiPC aggregates into dopaminergic neuron was attempted by using the EZSPHERE™ continuously throughout a series of steps from the hiPSC aggregate-formation to induction of midbrain dopaminergic neuron.
(B) Phase-contrast images of a time course of 12 days.
(C) Immunostaining for the midbrain progenitor marker FoxA2 and neural marker βIII-tubulin at day 12.
(D) Flow cytometry analysis with Oct3/4 antibody indicated that there were almost no iPSCs remained without differentiation.
(E) Immunostaining for the TH, Tyrosine hydroxylase and relative value of dopamine secretion in Low and High-KCl measurements by ELISA.
2) Cardiomyocyte differentiation in EZSPHERE
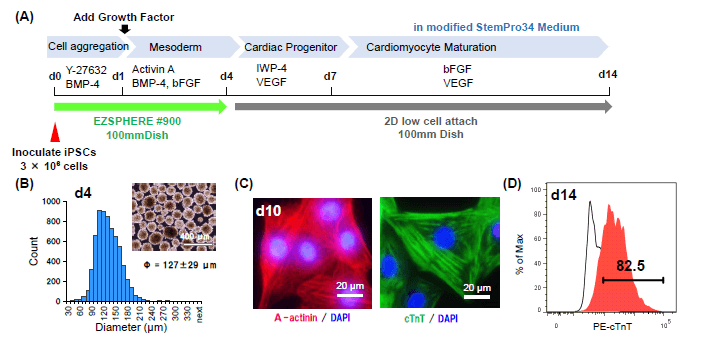
(A) Culture scheme. Cell aggregates were cultured in EZSPHERE™#900 (100mm Dish) for 4 days, and transferred 2D low cell attach dish after medium change.
(B) The size and microscopic image of Cell aggregates harvested at day 4.
(C) Immunostaining of, dissociated and re-plated cells at day 10 after day 1 for cardiomyocyte-specific maker α-actinin and Cardiac Troponin T, cTnT. The majority of cells had cardiomyocyte-specific structures, sarcomere.
(D) Flow cytometry analysis shows over 80% cells were positive for cTnT.
How to use EZSPHERE™ VIDEOs
Watch the videos below to learn how to use EZSPHERE™
| 1) How to seed cells in EZSPHERE™ vessel | 2) How to replace half of spent medium with fresh medium in EZSPHERE™ vessel | 3) How to replace total spent medium with fresh medium in EZSPHERE™ vessel (for beginners) |
| 4) How to replace total spent medium with fresh medium in EZSPHERE™ vessel | 5) How to collect spheroid from EZSPHERE™ vessel |
Download
Referred protocol / References
Protocol: iPS spheroid formation in EZSPHERE devices
References:
- Hiroyuki Koike, Ran-Ran Zhang, Yasuharu Ueno, Keisuke Sekine, Yun-Wen Zheng, Takanori Takebe, Hideki Taniguchi Nutritional modulation of mouse and human liver bud growth through a branched-chain amino acid metabolism Development 2017 144: 1018-1024; doi: 10.1242/dev.143032
- Waseem K. Raja, Alison E. Mungenast, Yuan-Ta Lin, Tak Ko, Fatema Abdurrob, Jinsoo Seo, Li-Huei Tsai Self-Organizing 3D Human Neural Tissue Derived from Induced Pluripotent Stem Cells Recapitulate Alzheimer’s Disease Phenotypes PLOS ONE 11(9): e0161969. doi: 10.1371/journal.pone.0161969
- Ayako Aihara, Natsuki Abe, Koichiro Saruhashi, Tatsuro Kanaki, Taito Nishino Novel 3-D cell culture system for in vitro evaluation of anticancer drugs under anchorage-independent conditions Cancer Science Volume 107, Issue 12 December 2016 Pages 1858–1866 DOI: 10.1111/cas.13095
- Taku Nakayama, Shimpei Otsuka, Tatsuya Kobayashi, Hodaka Okajima, Kentaro Matsumoto, Yuichiro Hagiya, Keiji Inoue Taro Shuin, Motowo Nakajima, Tohru Tanaka, Shun-ichiro Ogura Dormant cancer cells accumulate high protoporphyrin IX levels and are sensitive to 5-aminolevulinic acid-based photodynamic therapy Scientific Reports 6, Article number: 36478 (2016) doi:10.1038/srep36478
- Ryohichi Sugimura, Deepak Kumar Jha, Areum Han, Clara Soria-Valles, Edroaldo Lummertz da Rocha, Yi-Fen Lu, Jeremy A. Goettel, Erik Serrao, R. Grant Rowe, Mohan Malleshaiah, Irene Wong, Patricia Sousa, Ted N. Zhu, Andrea Ditadi, Gordon Keller, Alan N. Engelman, Scott B. Snapper, Sergei Doulatov, George Q. Daley Haematopoietic stem and progenitor cells from human pluripotent stem cells Nature volume 545, pages 432–438 (25 May 2017) doi:10.1038/nature22370
Application references of AGC tissue culture products Sep. 2015 - Hiroki Sato, Alimjan Idiris, Tatsuaki Miwa, Hiromichi Kumagai
Microfabric Vessels for Embryoid Body Formation and Rapid Differentiation of Pluripotent Stem Cells
Scientific Reports 6, Article number: 31063 (2016) doi:10.1038/srep31063 - Matsuura K, Seta H, Haraguchi Y, Alsayegh K, Sekine H, Shimizu T, Hagiwara N, Yamazaki K, Okano T
TRPV-1-mediated elimination of residual iPS cells in bioengineered cardiac cell sheet tissues
Scientific Reports 6, Article number: 21747 (2016) doi:10.1038/srep21747 - Aikawa N, Suzuki Y, Takaba K
A Simple Protocol for the Myocardial Differentiation of Human iPS Cells
Biological and Pharmaceutical Bulletin Vol. 38 (2015) No. 7 Pages 1070-1075 - Shimada H, Hashimoto Y, Nakada A, Shigeno K, Nakamura T
Accelerated generation of human induced pluripotent stem cells with retroviral transduction and chemical inhibitors under physiological hypoxia
Biochemical and Biophysical Research Communications, Volume 417, Issue 2, Pages 659-664 - Zhang RR, Takebe T, Miyazaki L, Takayama M, Koike H, Kimura M, Enomura M, Zheng YW, Sekine K, Taniguchi H
Efficient Hepatic Differentiation of Human Induced Pluripotent
Stem Cells in a Three-Dimensional Microscale Culture Stem Cells and Tissue Repair Methods in Molecular Biology Volume 1210, 2014, pp 131-141
Ordering Information
#900 Diameter: 400-600 µm, Depth: 100-300 µm
| Product | Size | Storage | Cat.No. | PKG Size | Price | |
|---|---|---|---|---|---|---|
| EZSPHERE™ 35 mm Dish, Type 900 | No. of Well: approx. 2,700/dish | Room Temp. | 4000-900SP | 10 dishes | 473.00 | Buy |
| EZSPHERE™ 60 mm Dish, Type 900 | No. of Well: approx. 6,500/dish | Room Temp. | 4010-900SP | 10 dishes | 506.00 | Buy |
| EZSPHERE™ 100 mm Dish, Type 900 | No. of Well: approx. 17,000/dish | Room Temp. | 4020-900SP | 10 dishes | 539.00 | Buy |
| EZSPHERE™ 6-well Plate, Type 900 | No. of Well: approx. 2,700/well | Room Temp. | 4810-900SP-N | 5 plates | 291.00 | Buy |
| EZSPHERE™ 24-well Plate, Type 900 | No. of Well: approx. 470/well | Room Temp. | 4820-900SP | 5 plates | 291.00 | Buy |
| EZSPHERE™ 96-well Plate, Type 900 | No. of Well: approx. 100/well | Room Temp. | 4860-900SP | 5 plates | 291.00 | Buy |
#901 Diameter: 250 µm, Depth: 100 µm
| Product | Storage | Cat.No. | PKG Size | Price | |
|---|---|---|---|---|---|
| EZSPHERE™ 35 mm Dish, Type 901 | Room Temp. | TCI-4000-901SP | 10 dishes | - | Inquire |
#902 Diameter: 450 µm, Depth: 200 µm
| Product | Size | Storage | Cat.No. | PKG Size | Price | |
|---|---|---|---|---|---|---|
| EZSPHERE™ 35 mm Dish, Type 902 | No. of Well: approx. 2,700/dish | Room Temp. | 4000-902SP | 10 dishes | - | Inquire |
#903 Diameter: 950 µm, Depth: 400 µm
| Product | Size | Storage | Cat.No. | PKG Size | Price | |
|---|---|---|---|---|---|---|
| EZSPHERE™ 35 mm Dish, Type 903 | No. of Well: approx. 1,300/dish | Room Temp. | 4000-903SP | 10 dishes | 484.00 | Buy |
| EZSPHERE™ 6-well Plate, Type 903 | Room Temp. | TCI-4810-903SP-N | 5 plates | 292.00 | Buy | |
| EZSPHERE™ 12-well Plate, Type 903 | Room Temp. | TCI-4815-903SP | 5 plates | 440.00 | Buy | |
| EZSPHERE™ 12-well Plate, Type 903 | Room Temp. | TCI-4815-903SP-10P | 10 plates | - | Inquire | |
| EZSPHERE™ 12-well Plate, Type 903 | Room Temp. | TCI-4815-903SP-50P | 50 plates | - | Inquire | |
| EZSPHERE™ 96-well Plate, Type 903 | Room Temp. | TCI-4860-903SP-10P | 10 plates | - | Inquire |
#904 Diameter: 1000 µm, Depth: 350 µm
| Product | Size | Storage | Cat.No. | PKG Size | Price | |
|---|---|---|---|---|---|---|
| EZSPHERE™ 35 mm Dish, Type 904 | No. of Well: approx. 700/dish | Room Temp. | 4000-904SP | 10 dishes | - | Inquire |
| EZSPHERE™ 6-well Plate, Type 904 | Room Temp. | TCI-4810-904SP | 5 plates | 292.00 | Buy |
#905 Diameter: 1,600 µm, Depth: 800 µm
| Product | Size | Storage | Cat.No. | PKG Size | Price | |
|---|---|---|---|---|---|---|
| EZSPHERE™ 35 mm Dish, Type 905 | No. of Well: approx. 260/dish | Room Temp. | 4000-905SP | 10 dishes | 484.00 | Buy |
- The shipping method is FedEx Ground or equivalent service.
- Products starting with "TCI" as Cat. No. are custom order items. Please feel free to contact us to inquire about pricing and lead time.
