Application : Comparison of biphenyl and other aromatic stationary phases
Many of our columns feature aromatic ring-based stationary phases with strong π-π interactions, notably our πNAP (naphthylethyl) and PYE (pyrenylethyl) columns. Several other companies produce similar columns using a biphenyl phase, which has become a popular alternative to C18 in recent years. Here, we compare these columns for several kinds of analysis.
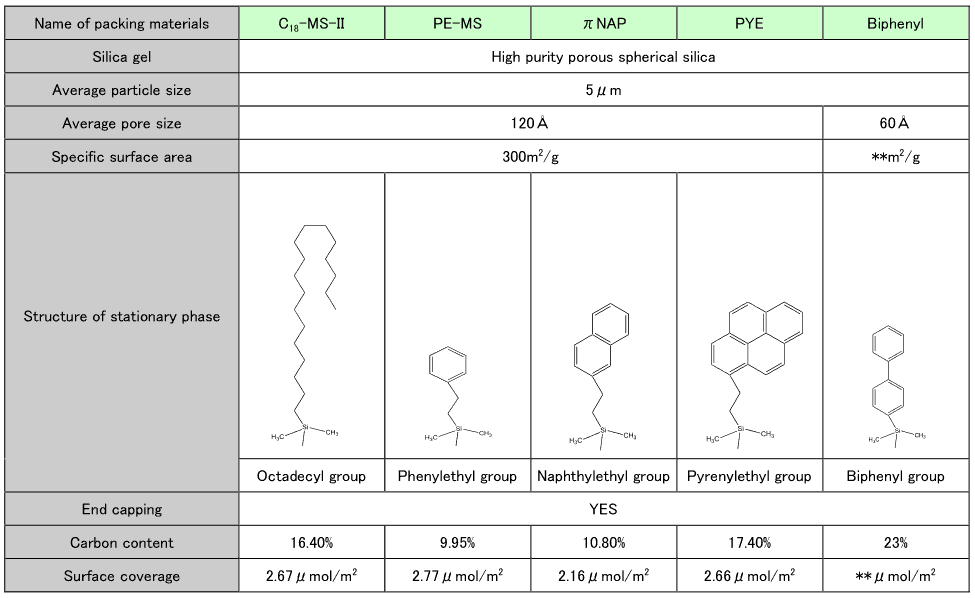
- C18-MS-II, PE-MS, πNAP, and PYE are made by Nacalai Tesque under the COSMOSIL brand
Analysis conditions
| Column size: | 4.6 mm I.D. x 150 mm |
| Mobile phase: | Listed in each figure |
| Flow rate: | 1.0 mL/min |
| Detection: | UV 254 nm |
| Temperature: | 30°C |
Evaluation of chromatographic properties
1. Comparison of hydrophobic selectivity
Each stationary phase was evaluated for hydrophobic selectivity by evaluating the separation factor of benzene and toluene.
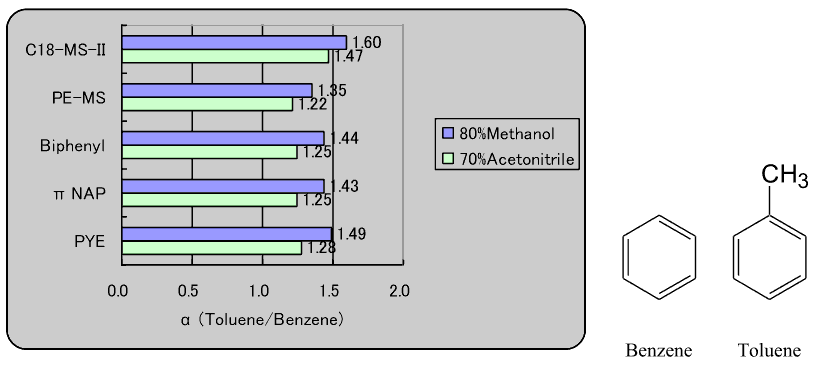
In order of hydrophobic selectivity: C18-MS-II > PYE > πNAP, biphenyl > PE-MS.
2. Comparison of π-π interaction
Each stationary phase was evaluated for π-π interaction strength by evaluating the separation factor of benzene and nitrobenzene. Methanol and acetonitrile solutions were used as mobile phases.
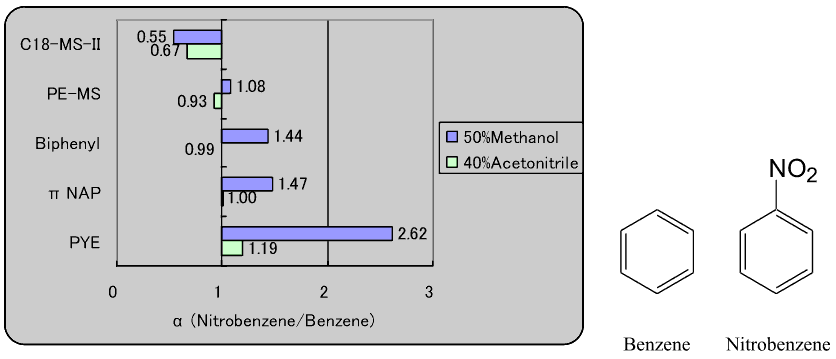
In general, π-π interaction becomes stronger as the number of aromatic rings in the stationary phases increases. In addition, the interaction strength of the biphenyl column, which has two rings connected by a single bond, and πNAP, which has two fused rings, was nearly the same.
The methanol-based mobile phase showed much higher figures than acetonitrile. As acetonitrile has its own π electrons, it is thought that it interferes with the π-π interaction between the sample and the stationary phase.
3. Improvement of analyses using aromatic stationary phases
(1) Berberines
πNAP and biphenyl perform similarly.
(2) Tocopherols
πNAP and biphenyl perform similarly. PYE offers greatest separation.
(3) Positional isomers of tolunitrile
πNAP significantly improves on both C18 and biphenyl.
(4) Phthalonitriles
πNAP significantly improves on both C18 and biphenyl.
(5) Nucleobases and Nucleosides
πNAP can separate more of the sample compounds than biphenyl.
(6) Adrenal cortical hormones
πNAP significantly improves on both C18 and biphenyl.
(7) Sterols
PYE improves on both πNAP and biphenyl.
(8) Sulfone selectivity
Biphenyl performs the best, although all columns were able to separate the compounds.
4. Conclusions
- Hydrophobic selectivity and π-π interaction strength were compared for aromatic stationary phases.
- π-π interaction strength increased with the number of aromatic rings in the stationary phase.
- The biphenyl and π-π stationary phases had similar π-π interaction strength.
- πNAP offered improved separation in many different analyses.
- By increasing the strength of the π-π interaction, it was possible to separate compounds that could not be done on C18 and conventional phenyl columns.








